Have you ever thought about how preparing food is similar to chemistry? Would you want to know how they are similar? You take whole ingredients and you put them together to make a brand new dish; you take elements and put them together to make a compound. In an even broader perspective, everything is made up of chemicals! The air we breathe, the water we drink, the food we eat.. and in that case, this piece is about what really goes into making the food we eat every single day, and how chemistry is intertwined with it.
I will be discussing six different ways in which cooking relates to chemistry; the first topic of which is going to be the differences in chemical and physical changes in chemistry, and in preparing food. To start, a physical change is a change in the state of matter. There are three main states of matter: you know these! Solid, liquid, and gas. Let’s say you want to make a Mac ‘n cheese dish. When you take a block of cheese and grate it up, the cheese doesn’t change. Although the shape is different, that cheese is still the same, just in a different state of matter. Another example of a physical change is ice cream melting. Maybe it’s a hot day and your ice cream melts; that ice cream hasn’t changed! It is just in a liquid state instead of a solid. In chemistry, you can take water and put it into a freezer, and it is still water. Maybe you melt mercury in a lab; although it’s in a liquid state, it is still the same metal. Now when it comes to chemical changes, there is a little more of a transformation. If a chemical change occurs, the starting product is not the same after. If you ever flip over a pancake, or cut into a cake and see bubbles, that means gas was produced, indicating a chemical change happened. Other things like smell could tell you a chemical change has happened with your food. The smell of sour milk or moldy cheese tells you a chemical change has happened. In chemistry, a chemical change would be burning wood. It starts as wood, but ends in ash, carbon dioxide, nitrogenous gases, and more.
The second topic of discussion is converting between measurements, whether it be moles and atoms or grams and teaspoons. We all know that when you have a recipe, you have to follow the measurements to ensure good results come out of it. If a recipe calls for 200 grams of sugar, how many pounds should you buy from the store? How many teaspoons are in a 1/4 cup? Those are all questions that could easily apply to chemistry, too. How many atoms are in a mole? How many grams are in an atom? For both cooking and chemistry, you can use a conversion factor diagram to solve these. 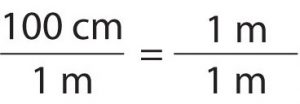
The third topic of discussion is all about comparing properties, whether it be metals vs. metalloids, or temperature vs. moisture. In cooking and baking, determining the physical properties of your food is important. What is the color? How is the texture? What is the boiling point for certain foods? All of these things play a role in making your perfect dish, as they also do in chemistry. If you want to produce certain compounds of elements, how would you do that? Well, by determining the properties. On the periodic table, every element is either a metal, nonmetal, or metalloid. These properties tell you whether you can bond them together or not.
The fourth topic is about identifying the differences between ionic, covalent, and metallic bonding in chemistry. But did you know these bonds occur in cooking as well? In chemistry, elements bond to each other based on how many electrons they can share, or take. When an ionic bond occurs, it is because of the electrostatic attraction between oppositely charged ions, and they then transfer electrons to each other. When a covalent bond occurs, two atoms share electron pairs between each other. These elements bond together to create new substances. When you’re preparing food, simple things like salt and water contain chemical bonds! Salt, which is made up of sodium and chloride, contains an ionic bond to create the table salt we know and love. Biochemistry also includes loads of information on how the building blocks of all food are simple chemical compounds!
The fifth topic is all about calculations, whether it be calculating percentages of each element in different compounds, or calculating how much flour to put in your pizza dough mixture. It is very important that when calculating chemical composition, you get your formula right. By finding out the percentages of elements in different compounds, we can then determine the empirical formula from those numbers. The empirical formula simply means a chemical formula reduced all the way down to its smallest state. With glucose, the formula is C6H12O6, but the empirical formula of that is CH2O. By dividing each subscript by two, you’ve found the empirical formula of glucose! Now, with cooking, finding out the percentage of every ingredient that goes into something you make is extremely valuable. The wrong amount of yeast in your dough and the whole thing turns to mush; or adding too much oil into your brownie mixture and that leaves it in a liquid state out of the oven. Overall, calculating your measurements of each chemical you put into a compound, whether that is in a lab or in a kitchen, is important.
The sixth and final topic for today is chemical reactions! These are essential in both chemistry and cooking. In chemistry, combining different elements together forms many different reactions. One I want to focus on is a combustion reaction. A combustion reaction occurs when a substance reacts with oxygen gas, releasing energy in the form of light and heat. For example, when methane burns in oxygen, it releases carbon dioxide and water. In cooking, there are hundreds of ways to use chemical reactions to make something new. A few great examples of these are fermentation, caramelization, and the Maillard reaction. The Maillard reaction is the chemical reaction that occurs in the presence of heat between amino acids and reducing sugars that results in food browning.
Overall, just from this information, you can realize that chemistry is infused in everything we do! Whether it’s melting ice cream, converting measurements, finding the boiling point of foods, determining what type of chemical bonds occur in salt, calculating percentages, or the process of browning food, every single aspect links to chemistry. With this information, we can realize that science is connected to everything, and taking advantage of that can produce great things in our lifetime.

























